Eliminate Your Controlled Substance Paperwork
Replace your paper narcotic logs with DEA-compliant electronic reports…
The DEA requires detailed logs for all entities that handle controlled substances. MedServe allows you to replace your paper logs with digital records creating several major benefits:
As the DEA requires entities to keep their records for at least two years, that storage of paper data can get bothersome
Retrieving and sharing data is incredibly efficient and fast so you can provide auditors with the exact dates of activity that they request
The electronic records can’t be tampered with
There have been multiple cases within the last few years of staff at healthcare facilities diverting controlled substances for personal use and altering or destroying paper logs to cover their tracks
Eliminate DEA compliance headaches, go digital with MedServe…
Cloud Reporting Capability Benefits
Records automatically updated in real-time
Protects from record tampering
Records all necessary information for wasting medications
Efficiently maintains records of which med is going to which specific patient or destination
DEA-compliant
Staff and users can add notes for any transaction that integrate neatly into the records
All necessary counts and reconciliation processes can be recorded within your electronic records
Records can be printed or exported in Word, PDF, or Excel formats
All data is stored in the cloud, so you can access your reports from any web browser
Your Options When Selecting a Controlled Substance Cabinet
In the straightforward sense, you have essentially two options: a cheap solution (a traditional key-locked narcotic cabinet) that meets the bare minimum of your needs or a very expensive digital solution (like Pyxis) that’s difficult to justify the price for you
The cheapest solution is a key-locked cabinet, which you can add very rudimentary staff tracking by adding a digital keypad. However, either way, you’re going to need to rigorously record all of your controlled substance activity on paper records and keep those records for multiple years.
On the other side of the pendulum, you have very expensive solutions that will likely cost a minimum of $30,000 but can easily go up significantly more than that. Mainly, this stems from the fact that most digital controlled substance cabinets, otherwise known as automated dispensing cabinets, are built for hospitals. They’re robust, with significant benefits that come at a hefty price. For example, a single medication storage unit found in a hospital can easily cost $100,000. But hospitals can afford the hefty price, and on top of that, they need all the digital sophistication because of the amount of staff and drugs that they’re handling. But for most facilities other than hospitals, these types of cabinets just don’t make sense.
So for anyone who’s looking to upgrade from a key-locked controlled substance cabinet, the options are really slim. It’s incredibly difficult to find a system that allows you to eliminate all the required paperwork for controlled substances without going over budget. That’s precisely why we developed MedServe’s digital controlled substance cabinets to be the middle ground between a key-locked system and the digital solutions found in hospitals. MedServe’s digital controlled substance cabinets are an affordable upgrade to key-locked cabinets that provide the ability to eliminate your paperwork for controlled substances, while also allowing administrators to keep track of their drug inventory in real-time.
Modernize your Controlled Substance Record Keeping with MedServe
Controlled Substance Storage Requirements
There are a plethora of rules and regulations that come with storing and using controlled substances in your healthcare facility. This list is not in order of importance. MedServe’s cabinets satisfy all storage requirements for controlled substances.
Double-Locking Required
All controlled substances must be secured with dual access (double-locked)
If you’re using keyed access, the two locks must not be opened by the same key. Two separate keys are necessary
Keys can’t be stored in the open and should not be stored near the cabinet
Two keys should not be stored in the same location
When employees are terminated, they are not allowed to have continued access to the cabinet
For keys, they will need to turn in their keys. If any keys are lost or misplaced, the locks on the cabinet must be switched immediately
Numeric combinations or keypad PINs, need to be changed or updated
Scanned ID badges or key fobs, must be electronically disabled within the system
Securely Mounted
Cabinets less than 750 lbs. should be bolted or mounted to the wall, floor, or countertop
Access
Controlled substances may only be accessed by authorized personnel
Unauthorized personnel must not have any access to the controlled substances in any capacity
For example, if the controlled substances are in a single-compartment safe that’s shared with other supplies or non-controlled meds, an unauthorized staff member can’t access that safe in order to get those non-controlled items
Separating CS Schedules
Depending on state regulations, some schedules of controlled substances (I, II, III, IV, V) may need to be separated from others
Regulations from schedule to schedule do change for some of the record-keeping and storage requirements
Ex: Separating schedule II from schedules III-V
Separate compartments within a specific cabinet or safe are compliant
Completely separate lockers or cabinets for each different schedule of controlled substances are not required
Stop Controlled Substance Recordkeeping on Paper…
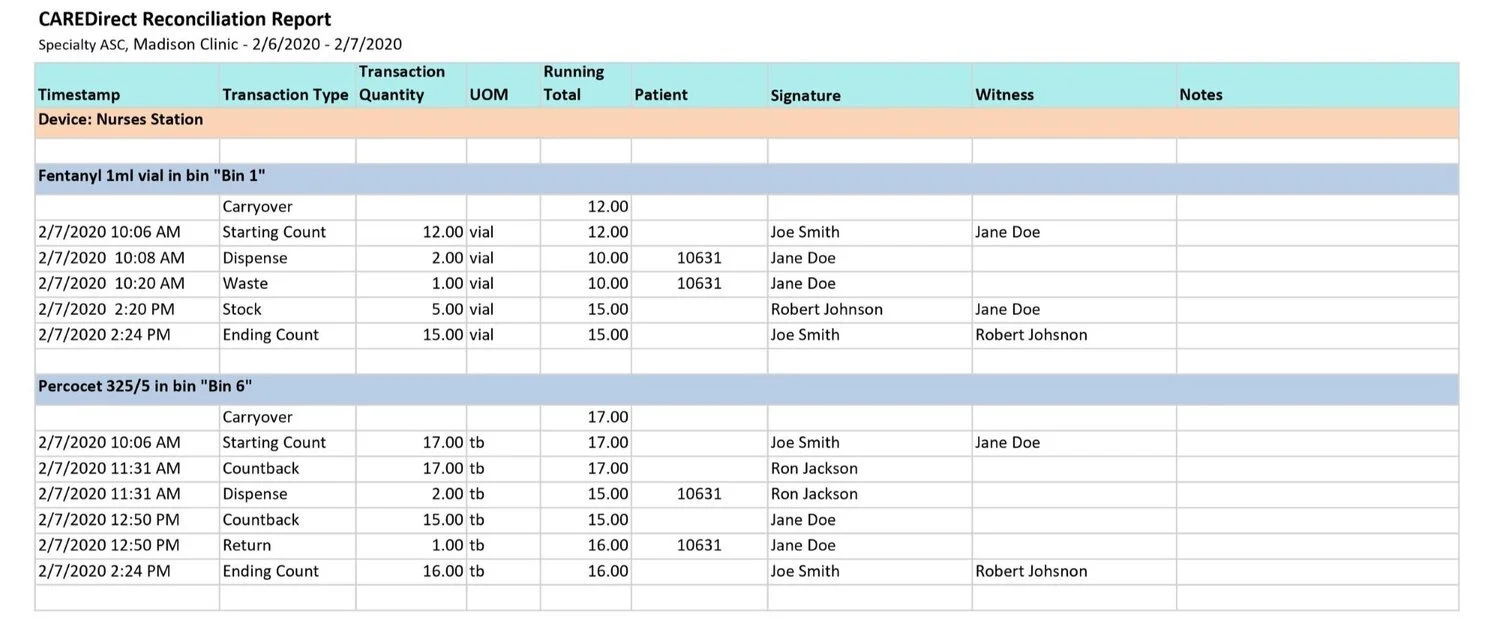
Instead of recording which staff member takes out a drug for each specific patient or other destination on paper, our system automatically records that data to build digital records in real-time. These records can be immediately accessed by an administrator. Paper records for controlled substances are usually pretty tight on space. There’s a lot of information that needs to fit on an 8.5 x 11-inch sheet of paper. Usually that leaves virtually no room to make notes or clarify any special circumstances for any particular incident. Sometimes an interaction with a controlled substance needs clarification. With our system, staff can make notes on any transaction that then shows up on the web-based reports. This helps immensely with record keeping as you don’t have to work within the constraints of a standard sheet of paper. Notes can be as long and descriptive as necessary.
Print or Export Controlled Substance Logs and reports as a PDF, or Excel
MedServe’s digital controlled substance records allow administrators to view activity across all cabinets for all staff members from any browser. You can see what specific type of activity each individual is performing (giving meds to a patient, wasting a med, etc.). You can check inventory levels for all of your drugs from your reports and see which daily reconciliations have not been performed. The reports can be easily exported as PDFs, Word documents, or Excel spreadsheets for audits. In addition, looking for and displaying specific dates or weeks of activity takes just a few easy clicks.
MedServe generates DEA-compliant records when staff interact with controlled substances.
These digital records can be accessed from any computer or tablet. MedServe’s software is continually updated to comply with DEA regulations, saving administrators from worrying about changes to the DEA's record keeping requirements.
DEA Controlled Substance Record Keeping Requirements
All records must be kept for a minimum of two years so that auditors have access to them when needed
DEA auditors often make unannounced visits to facilities where controlled substances to inspect your records and ensure your facility’s compliance with the Controlled Substance Act (CSA).
Violations of the regulations included in the Controlled Substance Act can result in increasing levels of penalty, including:
Letter of Admonition
$10,000 fine for each individual violation
Suspension or revocation of registration
Prison sentence
Store controlled substance logs separately from other medication records unrelated to DEA compliance.
Records must specify which staff member interacted with each particular controlled substance, the quantity used, the date and time of the interaction, and the specific destination of the substance (patient, etc.)
The DEA-compliant records should consistently indicate the running totals of each controlled substance from transaction to transaction
The records should also be securely stored and not easily accessed
Risks of tampering are always a concern when dealing with paper records
Daily counts of the controlled substances should be witnessed by another staff member
The witness should sign off on the transaction and their name should be recorded
Wasting and disposal of controlled substances should also be witnessed, with that witness on record as well
If records indicate that some controlled substances are not accounted for, submitting DEA Form 106 to authorities might be necessary
Require Count Backs
Identify inventory discrepancies quickly
MedServe allows you to turn on mandatory count backs for any medications stored in the cabinet. You can customize the system to require a count back whenever they access a bin with a controlled substance. For example, if a controlled substance shared a bin with something like Ibuprofen, the system would still require a count back even when someone was simply trying to access the Ibuprofen. This ability to turn this feature on and off allows you to help discover inventory discrepancies faster and identify exactly when the count began to be off. With a key-locked system, you can implement a count back policy, but in reality, you can’t be certain of who accessed the contents. You only know which staff members recorded their access on the paper logs. If someone ignored to write about their access on the paper log, there would be no record of it, unlike our system that always tracks each individual that accesses any specific bin in the cabinet.
Witnessed Reconciliation
Automate daily counts; receive immediate discrepancy alerts
The end-of-shift and start-of-shift counts are critical for any healthcare team that handles controlled substances. Our system allows your team to perform these two activities right on the cabinet with electronic signatures for each user who participates in the counts with required witnesses. Users sign in with a swipe of their ID badge and/or personal PIN code.
All discrepancies can automatically alert an administrator via email the instance the discrepancy is submitted.
Digital Controlled Substance Inventory Tracking
When accessing a MedServe bin containing a controlled substance, MedServe requires users to specify what they’re going to do with that particular med. Are they giving it to a patient, returning excess meds, wasting, or restocking? Here is a list of the records MedServe automatically records and stores in a tamper-proof digital format:
Dispense Transactions
Dispensing allows you to attribute the exact amount of a drug that a staff member takes to the specific patient that it’s intended for
You can electronically double-lock any MedServe bin with a controlled substance in order to require genuine two-factor authentication (ID badge and pin code)
Return Transactions
If someone took too much medication for a patient or surgery, they can bring it back, allowing the system to add it back into the inventory
Record Wasting Transactions
Wasting can be configured to require a second witness. The witness would use their personal ID badge or pin number to authorize the wasting so that there are two digital signatures on record
Restock
Restocking allows staff to add new drugs into the system so that they can be tracked